EqPA Annnounces New Customer Loyalty Program!
We are going to kick off the New Year with a real bang! Equine Partners America is pleased to announce a new Customer Loyalty Program which will operate on a quarterly basis, which is our way of saying thank you for being such great customers.
Every practice that orders 5 E-PET™ kits per quarter, beginning January 1 through March 31, 2013, will then be able to purchase their 6th E-PET™ unit for $180.00, a $200.00 savings! April 1st, the order minimum starts again.
As our users know, it makes sense to keep a spare kit on your shelf for that emergency case that you always seem to have. Of course, if you don't have a kit in hand, we certainly can get it to you on time, but it is costly and FEDEX seems the one who benefits most ...not us, you or your customers.
Many thanks to all our customers and new visitors to our booth at AAEP!
The winner of our free E-PET™ kit drawing was Dr. John Sangenerio from Dominion Equine Clinic. Dominion Equine Clinic is a progressive, full service equine veterinary practice serving South Hampton Roads, Northeastern North Carolina and the Virginia Eastern Shore. The practice has been proudly owned and operated by Dr. John Sangenario since 1984, and offers an alternative to transporting a horse or farm animal for selected surgery or hospitalization at a distant facility. It is a four man practice and they offer a full range of equine veterinary services. Congratulations Dr. Sangenerio and Dominion Equine Clinic!
Georgia Wet Lab a Successful AND Informative Evening!
On Monday evening, the 12th of November 2012, Dr. Jimmy C. Nash, kindly hosted a wet lab on the VetCell Stem Cell protocol and bone marrow aspiration techniques in conjunction with Equine Partners America, LLC. This session was conducted by Dr. Roger Smith, MA VetMB PhD DEO DipECVS MRCVS from the Royal Veterinary College in the United Kingdom, who first presented on his findings using the VetCell protocol over a 10 year period for SDFT injuries in race and sport horses. Dr. Smith then conducted a demonstration of bone marrow aspiration and stem cell ultrasound-guided implantation using the latest models of Vet Imaging's ultrasound equipment.

Dr. Jeffery Schaefer also presented on the popular E-PET™ product, the only true stall-side platelet offering in the market and the attendees got the opportunity to see how easy the product was to use and discuss the efficacy.

The wet lab was well attended and the attendees felt that the presentation and subsequent hands-on opportunity to practice ultra-sound aspiration of the sternum and injection techniques into the legs was extremely valuable. Many thanks to Dr. Jimmy Nash and his staff, as well as Keely Maginnis from Vet Imaging, Inc. for making this a successful, informative evening!

Olympic dressage horse Ravel has had stem cell therapy.
Did you see this article? (Click here to read) Olympic dressage horse Ravel has had stem cell therapy for a leg injury. Good to know that the top riders and top vets are starting to use stem cells more and more!!
Although the article is not completely correct in stating that he is the "first" Olympian to have stem cell therapy... VetCell has treated a horse that went to London 2012 and finished in a good position. Can't disclose who I'm afraid!
These state-of-the-art protocols may be performed locally by ambulatory veterinarians.

Dr. Alfredo Romero from Syracuse Equine gave our Hambletonian wet lab attendees a detailed presentation on VetCell's stem cell protocol and an easy to follow demonstration on aspirating bone marrow from the sternum and tuber coxae. Dr. Romero also demonstrated the new, non-invasive skin biopsy protocol from RenovoCyte for AutoCell EQ. Participants had the opportunity to practice their own skills at aspiration and implantation.
Participants were given the opportunity to discuss the new stem cell protocol, AutoCell EQ, with Dr. Shelly Zacharias of RenovoCyte . They also explored with Dr. Jeffery Schaffer from Pall Corporation the merits of their stall-side platelet solution, E-PET. These are the latest excellent offerings in the regenerative medicine arena.

Our presentation "stall" was packed with interested participants receiving updated information on stem cell and platelet uses for soft tissue injuries.
The key message from the session was that these state-of-the-art protocols can be performed locally by ambulatory veterinarians, thereby, saving their clients' time and money and providing leading-edge regenerative medicine in their own practices.
 New insights supporting the use of mesenchymal stem cell therapy as a treatment for superficial digital flexor tendinitis!
New insights supporting the use of mesenchymal stem cell therapy as a treatment for superficial digital flexor tendinitis!
Regardless of discipline, damage to the superficial digital flexor tendons is potentially career limiting to the horse as re-injury is common, occurring in approximately 56% of racehorses and 44% of non-racing sports horses (Dyson, 2004). Previous research has determined re-injury rates for racehorses treated using intralesional injections of bone marrow-derived culture-expanded mesenchymal stem cells (MSCs) (Godwin et al. 2012), yet no conclusions have been drawn regarding the non-racing sports horse.
Investigation of re-injury rates in the non-racing sports horse
Recent analysis of re-injury rates in non-racing sports horses treated for SDFT injury with intralesional injection of bone marrow-derived mesenchymal stem cell showed a significant reduction in re-injury rate when compared to historical controls treated using conservative management. Supporting earlier findings in racehorses that intralesional injections of MSCs offers an advanced level of healing compared to conservative management alone (Godwin et al. 2012).
The sample group for the study was obtained from the VetCell (UK) patient database (post-treatment follow up information was obtained independently). A total of 116 horses were identified and 71 were available for follow up. Inclusion in the study required horses to have been treated for core lesion to the SDFT using intralesional injection of bone marrow-derived mesenchymal stem cells before completing a rehabilitation period of up to one year including a controlled exercise programme, followed by two years back in full work - allowing for a period of a minimum of three years before follow up information was recorded. Of the original 71 horses, 68 matched the criteria for inclusion in the study. The influence of factors commonly associated with tendon injury with re-injury rates in horses treated using MSC therapy
Factors identified in the study as potential influencing variables included; time delay between treatment and injury, size of lesion, number of cells implanted and the age of horse at time of injury.
Data analysis found the relationship between time delay between injury and treatment and re-injury rate to be significant when horses were treated <44 days and >44 days post injury, supporting earlier reports (Fortier and Smith, 2008).
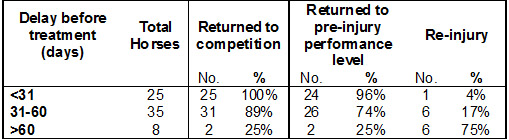
In addition, further analysis of the effect of time delay where the time periods were split into three groups (See Table 1). When treated <31 days following injury only 4% of horses re-injured compared to 75% when treated <60 days.
While no relationship was found linking re-injury rates and age of horse, size of lesion or number of cells implanted (likely due to the relatively small sample size) a possible implication of this finding is that MSC treatment may have the potential to overcome physiological attributes previously associated with increases in re-injury rates in horses treated using alternative methods.
Based on the current findings stem cell therapy should be considered as soon as SDFT injury is detected as any delay in sending off the bone marrow for MSC culture may jeopardise the success of the treatment.
EqPA & Pall Corporation sponsor Wet Lab

Equine Partners America, LLC and Pall Corporation sponsored a Wet Lab for CE credit at The Kentucky Equine Veterinary Spring Seminar on Thursday, April 26, 2012, at Lexington Equine Surgery and Sports Medicine, just one mile from the Kentucky Horse Park in Lexington, KY; and on Friday, April 27, 2012, at the Kentucky Horse Park in Lexington, KY, during the Rolex Kentucky Three-Day Event.
This seminar has been endorsed by the Kentucky Association of Equine Practitioners (15 CE hours credit). The focus of the seminar was on back and pelvic limb lameness, and included new technologies and research on the subject.
Equine Partners America, LLC and Pall Corporation hosted a lab entitled, "Protocols and Practical Experience in Aspiration Using The VetCell Stem Cell Procedure and E-PET™ Product Administration" on Thursday morning, April 26, 2012.
This four-hour wet lab began with a talk about the VetCell protocol history and technology, then moved on to practical application with the use of ultrasound on live horses to guide participants through the learning experience of aspirating bone marrow via the sternum. Participants were then provided the opportunity to practice this technique on their own with help from the lecturer of this session, Dr. Jimmy C. Nash.

Dr. Nash is the owner of Horner & Nash, DVM, PC, which is a four-veterinarian practice offering excellence in equine medicine, basic surgery, lameness, preventative medicine, reproduction, dentistry, nutrition and 24/7 emergency equine emergency care in the north Atlanta area.
The second half of the session was spent on administering the E-PET™ platelet product from Pall Corporation. This innovative product is completely stall-side and does not require the use of a centrifuge to get 7x platelet enhanced therapy.
This portion of the wet lab was conducted by Dr. Jeffrey Schaffer, Director Pall Life Science - Animal Health for New Initiative in Regenerative Medicine, Cell Therapy regarding Stem Cells, PRP and Prion Products, for PALL Life Sciences, Pall Corporation, East Hills, NY.
RenovoCyte LLC, Our Laboratory and Business Partner
RenovoCyte LLC, is our laboratory and business partner. As the US distributor of the VetCell protocol, with their proven stem cell technology for the treatment of tendon and ligament injuries, Equine Partners America (formerly VetCell America) have been using RenovoCyte as the laboratory for growing and maintaining this offering since March of 2011. We are now working with RenovoCyte on a new venture: their own protocol for the treatment of tendon and ligament injuries in equines, using skin as the collection medium for stem cell growth. Following is some background information on RenovoCyte and their business objectives from Dr. Zacharias, DVM, and Director of Veterinary Operations for RenovoCyte:
RenovoCyte is the veterinary brand of our parent company General BioTechnology, LLC. Our parent company is based in Indiana, has been in business since 1997, and is very well versed in multipotent mesenchymal stem cell biology and cryopreservation. We operate just outside downtown Indianapolis.The above provides a compartmentalized view of the work RenovoCyte has been doing that reflects the innovative and responsible company they are, with high praise coming from the FDA.
RenovoCyte strives to offer quality, responsible cell therapy, while offering an innovative and less invasive stem cell source for the animal. All of our products are cultured to a specific dose for the patient and contain viable cells. In the equine, we are able to obtain stem cells from tissue such as teeth (wolf teeth, molars, etc), placenta, and skin. We are also the processing laboratory for Equine Partners America, LLC, which utilizes the horse's bone marrow as the stem cell source via the VetCell process.
RenovoCyte's specific sources are meant to encourage collecting the tissue during routine procedures such as foaling or tooth extraction; the skin biopsy is available as the least invasive cell source. Once we have the tissue, we can either expand the stem cells and cryopreserve them in our liquid nitrogen vapor storage tanks (Wellness Banking®)or expand them for immediate use in the patient (cellular therapy). The majority of our treatments are given as a simple intravenous injection via an indwelling catheter, although we do now offer an intralesional injection for specific tendon/ligament injuries shipped in the horse's own PRP. RenovoCyte also offers autologous multipotent stem cell treatment for the canine, feline, and pet rabbit.
RenovoCyte was granted Investigational New Animal Drug (INAD's) trials from the FDA which allows us to conduct controlled clinical trials utilizing an allogeneic stem cell source in the horse, dog and cat. These trials involve various diseases and the purpose is to get a universal cellular therapy "drug" FDA approval which allows the veterinarian to keep a stock of cellular medicine at the veterinary clinic for intended use as needed. This would mean an easier process for the doctor and faster treatment for the animal, similar to a "blood bank" but would be an in-clinic, FDA approved "cellular therapy bank" for approved treatments.
If you would like to know more, please visit RenovCyte's website at www.renovocyte.com.
EqPA Participating in new Trials/Studies

2012 will be an exciting year for Equine Partners America, as we are working with our partners on getting several studies and trials off the ground.
The most important trial we are starting is on RenovoCyte's new stem cell protocol for tendon and ligament injuries. It will be using a unique and non-invasive collection method for stem cells using skin. We are looking for candidates for this trial and to get us started, the pricing is extremely attractive for the first 6 to 10 horses. So if you have a need and want to be part of a great new offering, please call us at 1-800-752-8538 to get the details on the protocol.
A Bleeders Study has already begun with RenovoCyte, and again, we are still looking for candidates for this study. We have had great results thus far. We would be happy to discuss the stem cell protocol that will be used in the study so again, if you have any candidates, please let us know. We will also begin a Laminitis Study with RenovoCyte, again using RenovoCyte's large dose stem cell protocol.
Finally, we continue to accept applicants for the trial involving platelet-rich plasma (PRP) and stem-cell treatment, which utilize Pall's Veterinarian Platelet Enhanced Therapy (E-PET) and VetCell stem cell protocol. This trial is dedicated to addressing soft-tissue injuries sustained in various disciplines, specifically to ligaments and tendons common in the equine athlete. Foremost stem cell authority Dr. Roger Smith participates in educational seminar hosted by Palm Beach Equine Clinic
Foremost stem cell authority Dr. Roger Smith participates in educational seminar hosted by Palm Beach Equine Clinic
We'd like to thank everybody for the wonderful turnout we enjoyed in Wellington on January 16, when Dr. Roger Smith spoke on "A Biological Approach to treating Tendon and Ligament Injuries". We'd like to thank Drs. Smith and Schaffer for their time and informative insights, as well as the entire staff of the Palm Beach Equine Clinic for their generous efforts. With over 30 equine practitioners attending the event, the feedback we have gotten has been tremendous.
Dr. Nash is the owner of Horner & Nash, DVM, PC, which is a four-veterinarian practice offering excellence in equine medicine, basic surgery, lameness, preventative medicine, reproduction, dentistry, nutrition and 24/7 emergency equine emergency care in the north Atlanta area.
 The second half of the session was spent on administering the E-PET™ platelet product from Pall Corporation. This innovative product is completely stall-side and does not require the use of a centrifuge to get 7x platelet enhanced therapy.
The second half of the session was spent on administering the E-PET™ platelet product from Pall Corporation. This innovative product is completely stall-side and does not require the use of a centrifuge to get 7x platelet enhanced therapy.
This portion of the wet lab was conducted by Dr. Jeffrey Schaffer, Director Pall Life Science - Animal Health for New Initiative in Regenerative Medicine, Cell Therapy regarding Stem Cells, PRP and Prion Products, for PALL Life Sciences, Pall Corporation, East Hills, NY.
Foremost stem cell authority Dr. Roger Smith to participate in educational seminar hosted by Palm Beach Equine Clinic
DAWSONVILLE, Ga. - Dr. Roger K. W. Smith, one of the world's leading authorities on stem cell technologies and their use in treating soft-tissue injuries, will be the keynote speaker of an educational seminar hosted by the Palm Beach Equine Clinic on January 16.
Dr. Smith is currently the Professor of Equine Orthopedics at the Royal Veterinary College in London, England. During the seminar he will spearhead discussion on specific innovations that have emerged to treat leg injuries in performance horses around the world. Entitled "A Biological Approach to Treating Tendon and Ligament Injuries," Dr. Smith's presentation will also detail emerging veterinary technologies that are being used to rehabilitate injured sport horses in all disciplines. Topics will include the epidemiology of tendon disease in the horse, the development of serological assay for tendonitis, and stem cell and platelet therapies for treating compromised tendons and ligaments.
Dr. Smith currently runs a specialist orthopedic service within the Royal Veterinary College. Through this service he directs a broad range of research projects into equine tendon disease. His primary area of research concentrates on the mechanisms of tendon aging, which extends to stem cell therapies developed in conjunction with a commercial endeavor, VetCell, he helped found.
The event is being sponsored by Equine Partners America, LLC, which was founded to supply products from the regenerative medical field to equine practitioners throughout North America. Equine Partners America focuses on veterinary products to help rehabilitate performance horses using VetCell and RenovoCyte Stem Cell Therapies and Veterinarian Platelet Enhancement Therapy (E-Pet) from Pall Corporation.
"We are quite pleased and excited to welcome Dr. Roger Smith to this educational event," said Moira McCracken, director of veterinary sales for Equine Partners America. "Dr. Smith is one of the world's foremost authorities in his field, and his expertise and insights into tendon injuries and stem cell treatments will be invaluable to everyone in attendance.Dr. Jeffrey A. Schaffer, Director of Pall Life Science-Animal Health for New Initiative in Regenerative Medicine, will also be a featured speaker during this one-night event. The event will be conducted at the White Horse Tavern in Wellington, Florida. It will commence at 6:30 pm and include dinner and a cocktail reception.
Dr. Nash is the owner of Horner & Nash, DVM, PC, which is a four-veterinarian practice offering excellence in equine medicine, basic surgery, lameness, preventative medicine, reproduction, dentistry, nutrition and 24/7 emergency equine emergency care in the north Atlanta area.
The second half of the session was spent on administering the E-PET™ platelet product from Pall Corporation. This innovative product is completely stall-side and does not require the use of a centrifuge to get 7x platelet enhanced therapy.
This portion of the wet lab was conducted by Dr. Jeffrey Schaffer, Director Pall Life Science - Animal Health for New Initiative in Regenerative Medicine, Cell Therapy regarding Stem Cells, PRP and Prion Products, for PALL Life Sciences, Pall Corporation, East Hills, NY.
Vet Cell participates in prestigious veterinary symposium in Lexington
Equine Partners, representing VetCell and Pall Corporation's E-PET™ product, was fortunate to participate in the Hagyard Equine Medical Institute's 13th Annual Bluegrass Symposium, held in Lexington, Kentucky on September 24-26.
Subtitled 'Equine Medical and Surgical Care for the Equine Veterinarian,' this prestigious event featured three days of seminars and sessions designed to share the newest veterinary medical technologies with equine practitioners from around the world. Vet Cell officials took part in several individual sessions, as well as an informative exhibit that allowed the approximately 100 participants to gain valuable insights into our full range of products. It was quite interesting to note that several authorities - such as Dr. David Wilson from the University of Missouri and Dr. Laura Werner from Hagyard - focused significant segments of their presentations on the emergence of regenerative medicine in equine veterinary practices. Dr. Werner in particular expounded on the many and various benefits that have been documented in studies conducted throughout the veterinary community. It's clear that methodologies such as platelet enhancement and stem-cell therapies are gaining widespread support by vets and equine caregivers in treating the performance horse.
It was quite interesting to note that several authorities - such as Dr. David Wilson from the University of Missouri and Dr. Laura Werner from Hagyard - focused significant segments of their presentations on the emergence of regenerative medicine in equine veterinary practices. Dr. Werner in particular expounded on the many and various benefits that have been documented in studies conducted throughout the veterinary community. It's clear that methodologies such as platelet enhancement and stem-cell therapies are gaining widespread support by vets and equine caregivers in treating the performance horse.
"The Hagyard conference was a wonderful event," said Anna Williams, chief financial officer for Equine Partners America LLC.
"The sessions covered many equine medical and surgical subjects, but I was interested to hear how many of the presenters discussed or alluded to regenerative medicine in their talks. We saw lots of interest in our E-PET™ product. Many of the vets were fascinated with the fact they don't have to carry around centrifuges and other equipment with our stall-side product. Many of the attendees were aware of this, and several were active users of the latest stem cell therapies. The equine veterinarian is becoming well informed and is expressing lots of interest in the latest developments in stem cells."
EqPA VetCell Americas to offer free consultation, product education during $1.5 million Hambletonian at Meadowlands
DAWSONVILLE, Ga. - Representatives of VetCell Americas, a leading veterinary technology company that specializes in equine stem-cell treatments, will be at Meadowlands on August 5-7 to offer free consultations and product education to horse enthusiasts attending the famed $1.5 million Hambletonian.
VetCell has long enjoyed a well established equine stem-cell business in the United Kingdom, and it recently expanded its operation into the United States through distributor Equine Partners of America LLC. VetCell officials will be present during Hambletonian festivities to promote stem-cell therapies in the treatment of equine leg injuries and lameness, as well as new technologies to combat exercise induced pulmonary hemorrhage, rhabdomyolysis (tying up), and osteoarthritis in the horse.  "The proliferation of stem-cell treatments and methodologies in horses is the most exciting advancement in veterinary technology in decades," said Moira McCracken, Director of Veterinary Sales for VetCell Americas. "We now possess quantifiable results that confirm stem-cell therapies can help performance horses from all disciplines return to successful careers. By introducing a proper stem-cell protocol, horses are now afforded 'second chances' they might not have enjoyed using outdated veterinary treatments. That's why my colleagues and I are here in New Jersey - to share this information with horse owners and enthusiasts and make sure they know what is now available to them."
VetCell recently announced the addition of an innovative intravenous (IV) product through its North American partner, RenovoCyte Cellular Medicine Laboratories.
"The proliferation of stem-cell treatments and methodologies in horses is the most exciting advancement in veterinary technology in decades," said Moira McCracken, Director of Veterinary Sales for VetCell Americas. "We now possess quantifiable results that confirm stem-cell therapies can help performance horses from all disciplines return to successful careers. By introducing a proper stem-cell protocol, horses are now afforded 'second chances' they might not have enjoyed using outdated veterinary treatments. That's why my colleagues and I are here in New Jersey - to share this information with horse owners and enthusiasts and make sure they know what is now available to them."
VetCell recently announced the addition of an innovative intravenous (IV) product through its North American partner, RenovoCyte Cellular Medicine Laboratories.
"The IV treatment is called Autocell-EQ, and it isolates multi-potent stem-cells from unique sources such as wolf teeth, skin biopsies, placenta and testes," said McCracken. "Those cells are preserved using RenovoCyte's patented Wellness Banking protocol or cultured to a known dose for autologous intravenous therapy. It's ground-breaking technology that we're excited about distributing to the thousands of people who care for horses in the U.S."
People interested in scheduling a consultation with VetCell representatives in New Jersey, or in getting more information about specific stem-cell products or treatments, can contact the company's corporate offices at 800-752-8538.
VetCell expands scope of stem cell therapies and treatments by teaming with innovative laboratory RenovoCyte
 DAWSONVILLE, Ga. - VetCell Americas, a leading veterinary medical technology company that specializes in the distribution of stem cell treatments and methodologies, is proud to announce a new professional collaboration with the innovative cellular laboratory RenovoCyte.
DAWSONVILLE, Ga. - VetCell Americas, a leading veterinary medical technology company that specializes in the distribution of stem cell treatments and methodologies, is proud to announce a new professional collaboration with the innovative cellular laboratory RenovoCyte.
VetCell has long enjoyed a well-established equine stem cell business in the United Kingdom, and it has recently expanded its operation into the United States through distributor Equine Partners of America LLC (EPA). VetCell specializes in the personalized service to veterinarians for the treatment of equine leg injuries and lameness.
As part of the strategic partnership RenovoCyte will be responsible for the processing of VetCell's mesenchymal stem cells (MSD) from equine bone marrow, as well as in-house veterinary support for VetCell's equine treatments in the U.S. Furthermore, RenovoCyte will now process and/or store pure stromal stem cells from bone marrow for VetCell's dedicated therapy for horses with injured tendons and ligaments. Once processed, these cellular treatments may be sent directly to veterinarians for injection into the patient's injured site.
"We are very excited by the new relationship VetCell has formed with RenovoCyte," said Moira McCracken, director of veterinary sales for EPA. "It's a wonderful opportunity for us to expand VetCell's functional capabilities and to draw on the strengths of both corporations. Most importantly, it will allow equine practitioners an unfettered platform to deliver the very best veterinary care for performance horses of all disciplines. It's truly a watershed moment for everyone involved".
As part of the partnership, VetCell will also now carry RenovoCyte's equine intravenous stem cell product, Autocell-EQ. This innovative stem cell technology allows for IV treatment of exercise induced pulmonary hemorrhage, rhabdomyolysis (tying up), and osteoarthritis in the horse.
"Autocell-EQ is one of the most cutting-edge products currently available in the spectrum of stem-cell treatments" said McCracken. "It isolates multi-potent stem cells from innovative sources such as the wolf teeth, skin biopsies, placenta and testes, then either cryopreserves the samples using their patented Wellness Banking protocol or cultures them to a known dose for autologous intravenous therapy. It's ground-breaking technology that we are excited about distributing to the thousands of people who care for horses in the U.S."
New Bundling Options from VetCell
Hoping everyone one had a great 4th of July and is now enjoying the full swing of summer!
Here at VetCell, we are striving to bring you current information on the latest stem cell therapies at the best price available. By bundling the pricing of our VetCell stem cell offering with cryo-storage at the lower price of $1,600, we hope more of your clients will take advantage of this combination to ensure lifelong protection against tendon and ligament injuries. Any future injuries can be treated with stem cells ready in storage to help with their recovery.
We are also working with veterinarians to discover the efficacy of using platelets and stem cells together; and now we are adding a new stem cell offering whereby a veterinarian can have 10M stem cells for injection today and come back for an additional 10M more within 14 days without cryo-storage. Further, we have bundled another offering for a veterinarian who may want to have that second injection from 15 days up to a year! During the North American Veterinarian Regenerative Medicine Conference this spring, there were many talks about the use of multiple stem cell injections in an injury-whether it was about the amount of stem cells used or the use of multiple injections, of course, dependent on the injury. We want to make sure our veterinarians have the capability to do whatever they deem necessary to get the animal on the road to recovery.
New Bundled Options:
Working with our innovative laboratory, RenovoCyte, you will see additional offerings being added in the coming months, but if you have a special need, please do not hesitate to let us know, as I am sure we can accommodate your requirements.